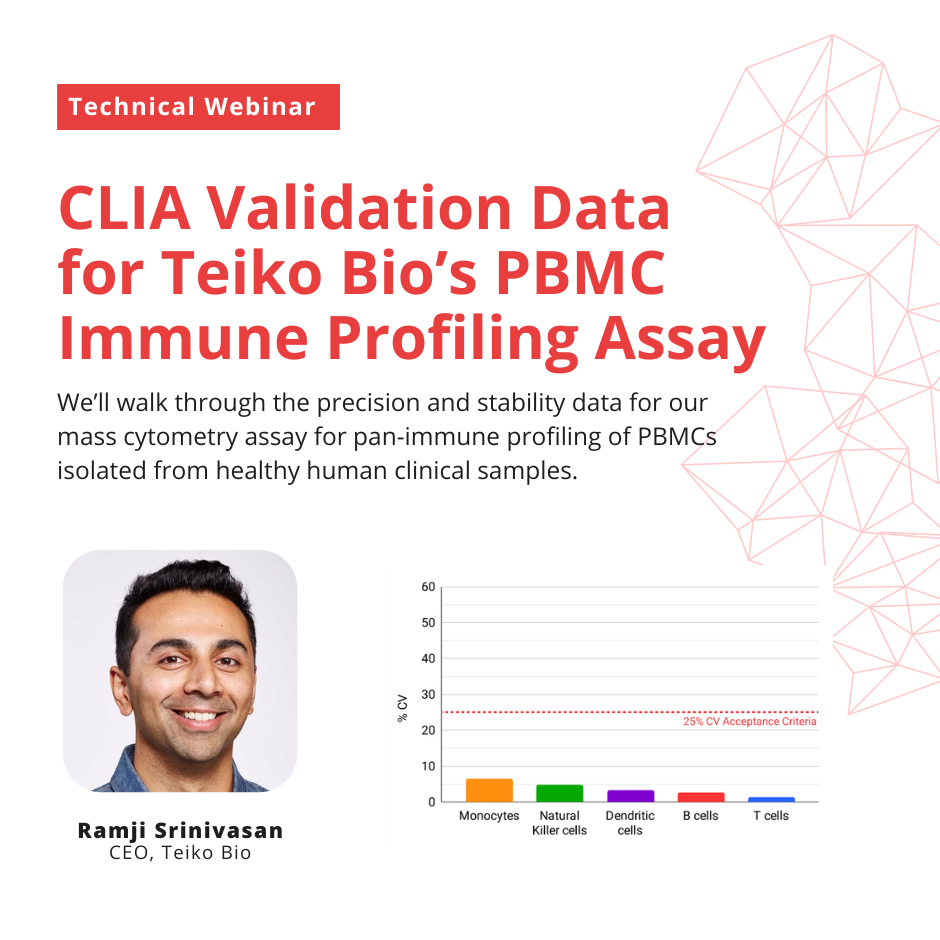
CLIA Validation Data for Teiko Bio’s Mass Cytometry Immune Profiling Assay for PBMCs
In this live webinar, Ramji Srinivasan, cofounder and CEO of Teiko Bio, will walk through the precision and stability data for our mass cytometry assay for pan-immune profiling of PBMCs isolated from healthy human clinical samples.