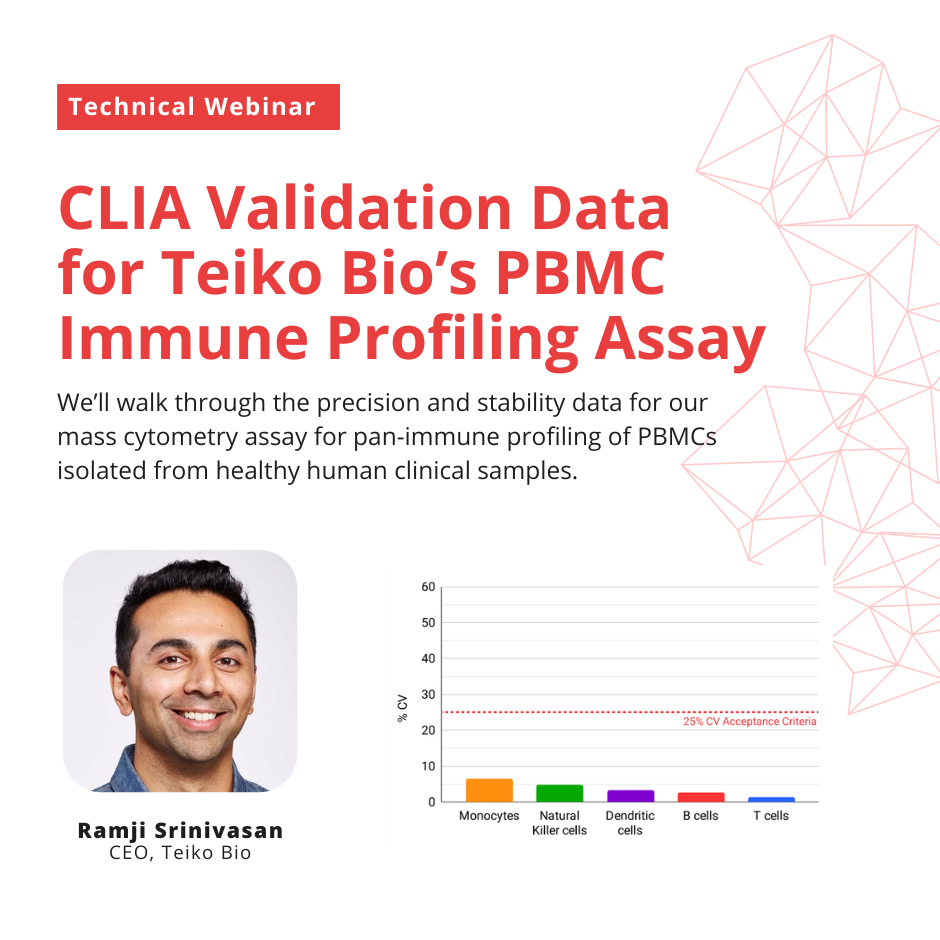
About the Webinar
In this live webinar, Ramji Srinivasan, Cofounder and CEO, and Kristina Magee, Associate Director of Lab Operations, will guide you through a full demo of the TokuKit, covering ever step from blood draw to kit processing and storage at -80°C. The TokuKit is designed for collection, fixation, and long-term preservation of whole blood samples, for cytometry analysis of longitudinal patient samples from multi-site clinical trials.
About the Speakers
Before Teiko, Ramji was Cofounder, CEO and Chairman of Counsyl, a women’s health genetic screening laboratory. Counsyl screened over 1M prospective parents, mothers-to-be and women at risk of hereditary cancer. In 2018, Counsyl was acquired by Myriad Genetics, Inc for $375M in cash and stock.
Ramji earned a B.S. in computer science and an M.S. in financial mathematics, both from Stanford University. Ramji also attended Stanford’s Graduate School of Business before dropping out to start Counsyl.
Prior to Teiko, Kristina Magee completed her MD as well as PhD in Cell Biology at the University of Bern, Switzerland. There, she investigated metabolic targets for the use in AML differentiation therapies. Following this, she held a role as a Postdoctoral Fellow at the University of Utah before joining Teiko in early 2024.