Being able to leverage the strengths and skill sets of a strategic partner can mean the difference between finding a therapeutic breakthrough in your research pipeline or not. Make the most of your time and resources by choosing an immune profiling partner who can advance your oncology, autoimmune, or infectious disease pipelines. Here are five things to look for when evaluating an immune profiling partner.
Page Anchor: #immunology-expertise – Not Visible On Front Immunology Expertise
It may seem obvious, but producing high-quality, interpretable data requires knowledge, skill, and experience. Be sure your immune profiling partner has enough experience to perform quality work in a timely manner and can troubleshoot challenges that arise. Does your service provider understand the nuances of different cell types? The subtleties of manual gating? Gating and computational expertise in high [40+] dimensional immune datasets can mean the difference between finding and missing subtle signals in your data.
How can you judge expertise when scoping a new partnership? Ultimately, the proof is in the pudding. The scientific leadership of the organization should have a trail of high-impact publications in established peer-reviewed journals and demonstrate innovative ways of understanding and exploiting our immune system to treat disease. Their team should be experienced and confident enough in the “language” of their technical knowledge base to creatively expand and grow its potential versus someone who only conventionally applies what has already been done before.
Another consideration is the technology being used to complete the immune profiling. The technique should make use of latest advances in science but at the same time must be properly vetted through the scientific process to ensure consistency and accuracy over time.
For example, mass cytometry or cytometry time of flight (CyTOF) is a technique that employs antibodies coupled to isotopically pure metals to detect and measure more than forty cellular features with single-cell resolution. It builds on the technology of flow cytometry to maximize resolution and parameterization (the number of markers that can be detected in a single cell). At the same time, the technique has been tried and tested and widely validated through rigorous research since becoming commercially available in 2009. Over the years, new techniques like SCAFFOLD [Single-Cell Analysis by Fixed Force- and Landmark-Directed] have been developed to visualize complex mass cytometry data. Powerful informatics techniques to make use of all the information mass cytometry data has to offer have been developed over the years by leaders in the field. Look for service providers that have expertise in the nuances of these different tools.
Page Anchor: #batch-effects-normalization – Not Visible On Front Batch Effects Normalization
Batch effects arise from non-biological factors that influence data results, masking the relevant biological data necessary to move the research project forward. By using a barcoding set methodology, separate samples can be grouped together and analyzed in one tube, minimizing the variation between sets, or batches, that can develop from uncontrollable technical fluctuations.1 Such technical variability can arise from slight changes in the calibration of equipment or in the implementation of experimental procedures, such as staining, that are unavoidable when each batch is analyzed.2
Equipment calibration fluctuations are detected and eliminated via normalization algorithms that are calculated based on the variation of standardized calibration control beads between batches, which would otherwise be identical.2 Likewise, the effects of slight differences in experimental technique, such as staining, are detected and eliminated using anchor, or reference, control samples.1,2 Technical and biological replicates applied to each batch are used as anchor samples. A single donor, cell line, or tissue sample could be used as an anchor sample depending on the details of the study and its objectives.1 By means of computer analysis, transformation factors are then calculated to normalize anchor sample variation between batches.2
Make sure your immune profiling company employs both instrument calibration controls and biological anchor samples to guarantee that data is normalized appropriately. Doing so ensures that biologically relevant information can be clearly detected and data between batches is comparable.
Page Anchor: #quality-control – Not Visible On Front Quality Control
Use of anchor, or reference, samples, calibration beads, and normalization algorithms are part of the broader picture of quality control. Quality control must be implemented for all components of an experiment including the instrument, reagents, methods, and samples.3 Instruments should be checked daily to make sure they are functioning properly within specified parameters, using calibration beads and diagnostic checks. Reagents must be tested for reliable performance, and methods must be consistent and standardized. Excellent quality control demands expert-level technical proficiency and problem-solving skills that only come from advanced training and experience.
Quality control extends to the handling and processing of your biological samples as well. Biological specimen samples are precious, and it is critical that your immune profiling partner make every effort to ensure that they are handled properly to generate reliable data you can be confident in. This includes quality control measures at every stage of sample processing, including on arrival, following fixation, and during data acquisition.
Regarding the handling of samples, disease stage and treatment can affect blood and PBMC (peripheral blood mononuclear cell) viability and quality. Thus, a good immune profiling partner is one who has experience processing many different types of biological specimens (e.g. whole blood, PBMCs, dissociated tumor or tissue samples, tumor-infiltrating lymphocytes, and bone marrow aspirate) from a variety of sources (human, mouse, non-human primates) collected using a variety of techniques (fixed and frozen).
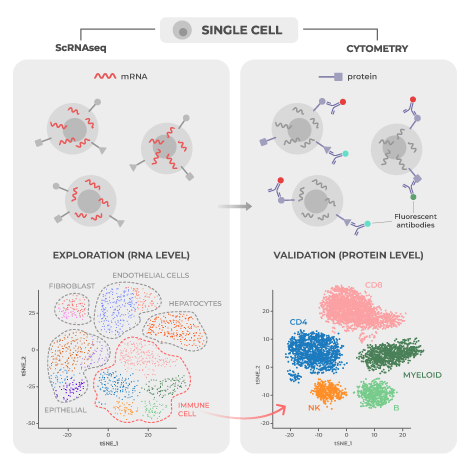
Page Anchor: #bioinformatics-pipeline – Not Visible On Front The complexity and volume of data from high dimensional single-cell analysis requires increasingly sophisticated computing technology. Many important informatics algorithms have been developed throughout the 2010s.
One of these is CLARA (Clustering LARge Applications) clustering for large cytometry datasets which allows the data to cluster into groups and can identify unique subsets that may be missed by conventional cytometry gating strategies. This can be paired with an algorithm like Cluster Identification, Characterization, and Regression (Citrus).7 This program enables detection of cellular characteristics associated with targeted outcomes, such as patient survival to identify statistically significant and biologically relevant biomarkers.
Another algorithm is called Single-cell analysis by fixed force- and landmark-directed (Scaffold) maps.7 It employs a technique known as force-directed graphs, where the amount of likeness between cells functions as an attractive force while groupings are repelled by a repulsive force. The power of this bioinformatics technique is its ability to provide a 2-dimensional reference map of the immune system with static landmarks that can be used for easy comparison between samples or conditions. Changes to the system can then be better interpreted in their proper context and evaluated in comparison to other research study outcomes.
You’ll want an immune profiling partner that is familiar with a diversity of bioinformatics pipelines and may have even been a part of their development and validation. Computational approaches continue to evolve and a partner with a strong foundation who is continuing to evaluate modern approaches will ensure confidence and longevity of your analysis.
Page Anchor: #turnaround-time – Not Visible On Front Turnaround Time
Time is precious and an important part of goal setting. When you assess turnaround time, it is important to look for a company that will provide end-to-end support at every step of the process from data acquisition through delivery and interpretation of results. Most contract research organizations (CROs) will cover the data acquisition, but leave the first mile and last mile up to you and your busy team. Look for a company that will work hand-in-glove with your existing scientific team to devise a detailed outline of the exact project scope, deadlines, and deliverables necessary to achieve your goals.
Leveraging the skill and experience of the right immune profiling partner to scope your project could save you a great deal of time and expense in the long run by avoiding errors that would stall your project or force your team to go back to the drawing board. Many CROs will leave you with raw data and excel spreadsheets. Look for a partner that provides data analysis and interpretation to save your company critical time and resources that could be better spent elsewhere.
Where to go for help
Whether you’re in the final stages of evaluating immune profiling partners or just beginning your search, our team of Senior Scientists is available to help answer any questions in your consideration process. You can book a meeting directly on our Contact Us page.