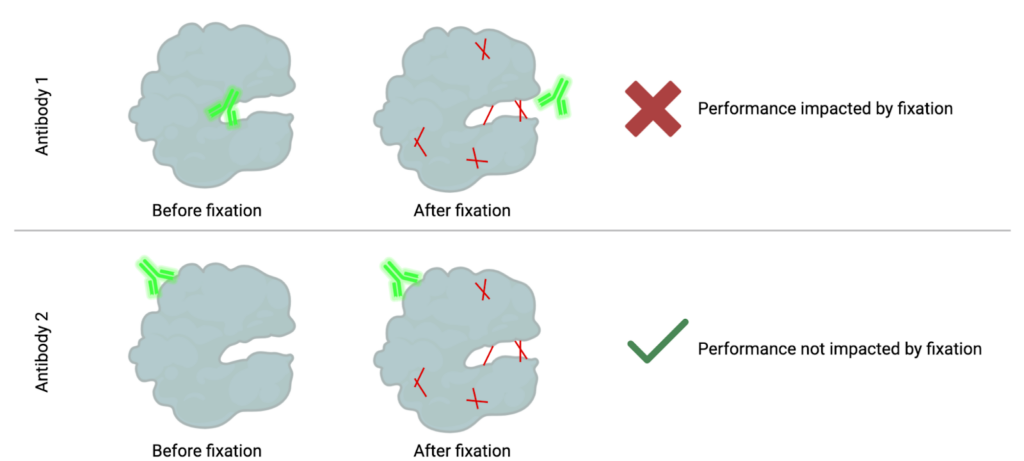
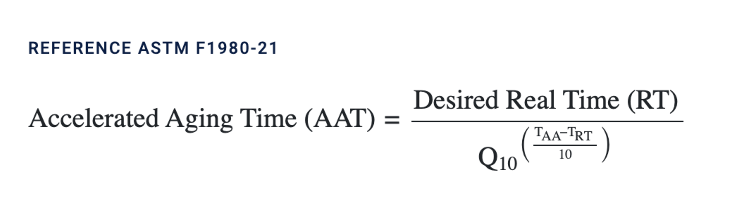How fixation can mask epitopes: When cells are fixed, the proteins get stuck together in different ways, making it harder for antibodies to reach and bind to the spots (epitopes) they normally would.
In both mass and flow cytometry, the goal is to measure specific immune populations. To measure these populations, we need to detect specific targets, often proteins, within individual cells. This detection relies on labeled antibodies binding to these targets, making them visible to the instrument. Antibodies bind to specific regions on a protein, known as epitopes. However, when samples are chemically fixed to stabilize them for storage, the fixation creates bonds (or methylene bridges) between nearby amino acids in a protein, a process known as cross-linking. These bonds, illustrated in red below, stabilize the protein but can block access to certain epitopes. As a result, some antibodies can no longer recognize their target because their binding region is now “masked.”
Fortunately, proteins have multiple epitopes, and other antibodies may bind to regions unaffected by fixation. These antibodies remain capable of detecting the protein in both fixed and unfixed states. Alternatively, fixation methods without formaldehyde such as methanol-based fixation can be utilized.
When working with fixed samples, it is essential to rigorously test each antibody in your panel to ensure its performance is not compromised by fixation. Proper testing helps ensure accurate and reliable detection of your targets in cytometry assays.