Matt Spitzer discusses his team’s recent publication in Cell
Immunotherapy ➡️ Lymph Node ➡️ Blood ➡️ Tumor
Even though immunotherapeutic drugs have been used in the clinic for more than a decade and have revolutionized cancer care, we don’t precisely know how they work. For a long time, scientists were unsure whether immunotherapy ‘revitalizes’ exhausted T cells inside the tumor to attack cancer anew or whether it recruits fresh T cells from elsewhere.
Today, a group of scientists led by Teiko cofounder and UCSF Associate Professor Matthew Spitzer published a new paper in Cell addressing this question. The study, co-authored by Rahim, Okholm, & Jones et al., reveals that T cells differentiate and multiply in the tumor-draining lymph nodes following immunotherapy, after which they circulate through the blood to reach the tumor.
T cells differentiate and multiply in the tumor-draining lymph nodes following immunotherapy, after which they circulate through the blood to reach the tumor.
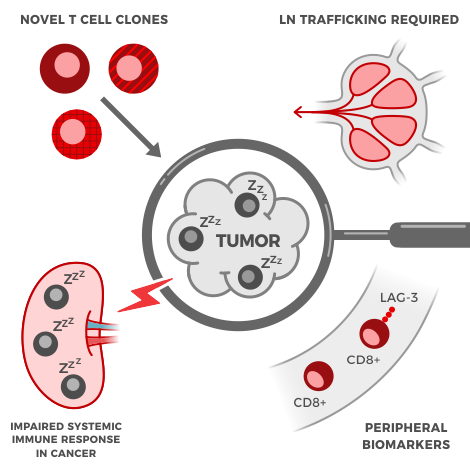
The authors used multiple single-cell technologies, including a 45-marker mass cytometry panel, to analyze immune profiles across tumor, blood, and tumor-draining lymph nodes from three cohorts totaling 25 patients with locally-advanced head and neck squamous cell carcinoma.
They first analyzed tumor and paired uninvolved (non-metastatic) lymph nodes collected at the time of surgery from a cohort of patients who did not receive immunotherapy. This revealed two clusters of self-renewing T cells that maintain antigen-specific responses in settings of chronic antigen exposure, such as cancer, called progenitor exhausted CD8+ T cells (Tpex). These Tpex cells were found in higher frequencies in the lymph nodes compared to the tumor.
After getting activated, Tpex differentiate into intermediate-exhausted (Tex-int) and terminally exhausted (Tex-term) CD8+ T cells. Using single cell RNA-sequencing and TCR-sequencing, the authors tracked unique T cell clones in both the tumor and lymph nodes. They discovered that Tpex in lymph nodes were clonally related to Tex-term cells found in the tumor microenvironment, suggesting that Tpex cells or their more differentiated progeny move from the lymph node to the tumor.
Tpex in lymph nodes were clonally related to Tex-term cells found in the tumor microenvironment, suggesting that Tpex cells or their more differentiated progeny move from the lymph node to the tumor.
The authors then sought to determine whether Tpex activation was taking place within the tumor or tumor draining lymph node following treatment with immunotherapy. Since recently activated T cells exit lymph nodes through the lymph, then enter the blood before trafficking into tissues, such as a tumor, they also asked whether Tpex or their differentiated progeny could be detected in blood as they traffic between tissues. To address this question, the authors analyzed blood samples from a separate cohort of 10 patients treated with atezolizumab prior to surgery. They collected blood at baseline (prior to treatment), at surgery (after 1-2 cycles of treatment) and at 1 month follow-up. At time of surgery, they found an increased proportion of proliferating Tex-int cells in the blood that correlated with Tpex and Tex-int frequencies in the lymph node. The authors did not see many proliferating T cells in the tumor itself, providing further evidence that these Tex-int cells probably came from the lymph node and were not already present in the tumor.
At time of surgery, they found an increased proportion of proliferating Tex-int cells in the blood that correlated with Tpex and Tex-int frequencies in the lymph node.
The study clarifies how immunotherapy works outside of the tumor, and highlights the importance of analyzing peripheral immune responses rather than just T cells in tumors. It also shows how immune profiling of blood can serve as a less invasive and more accurate readout of successful immune activation in the lymph node and tumor during immunotherapy.
Reference citation: Rahim MK, Okholm TLH, Jones KB, McCarthy EE, Liu CC, Yee JL, Tamaki SJ, Marquez DM, Tenvooren I, Wai K, Cheung A, Davidson BR, Johri V, Samad B, O’Gorman WE, Krummel MF, van Zante A, Combes AJ, Angelo M, Fong L, Algazi AP, Ha P, Spitzer MH. Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. (2023). Cell 186(6):P1127-1143.E18. doi: https://doi.org/10.1016/j.cell.2023.02.021
Graphical abstract, unedited and scaled for size, provided under Creative Commons (CC-BY-4.0) license.