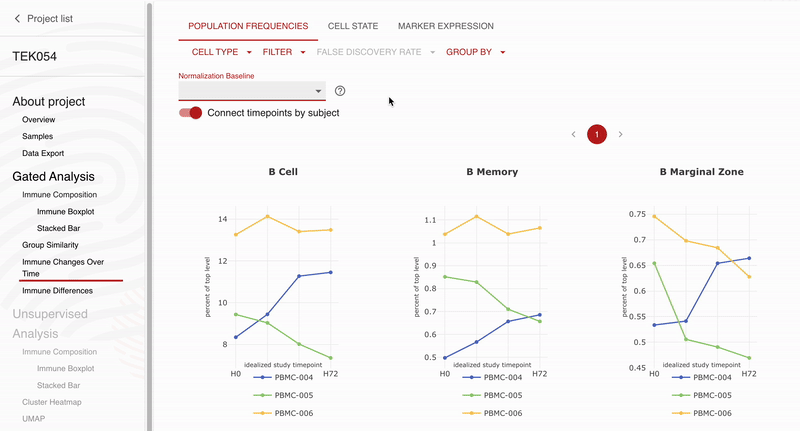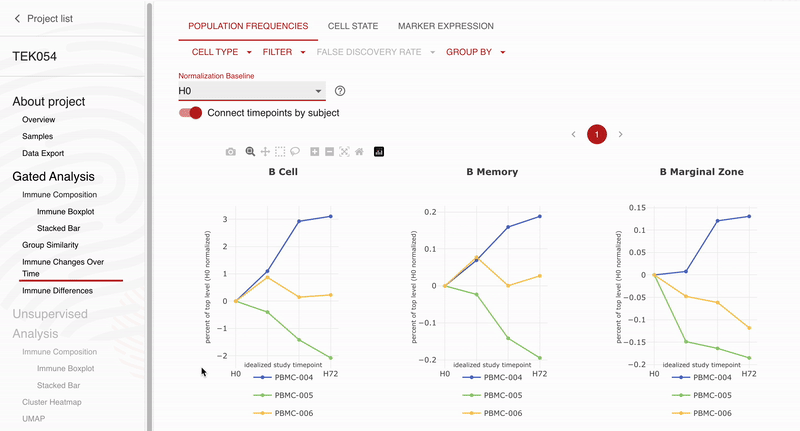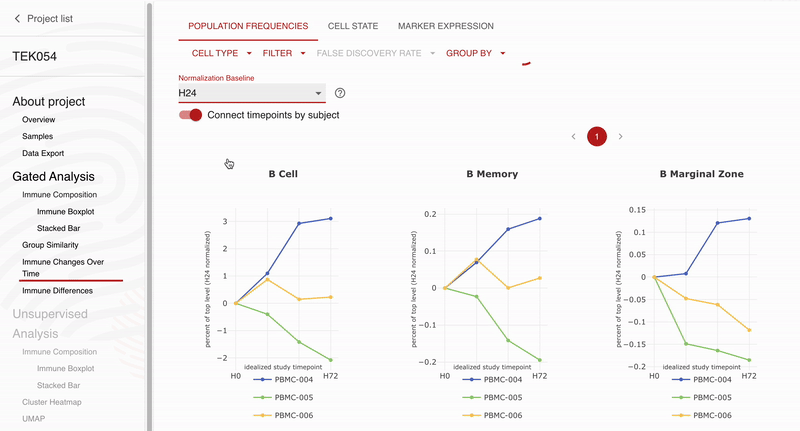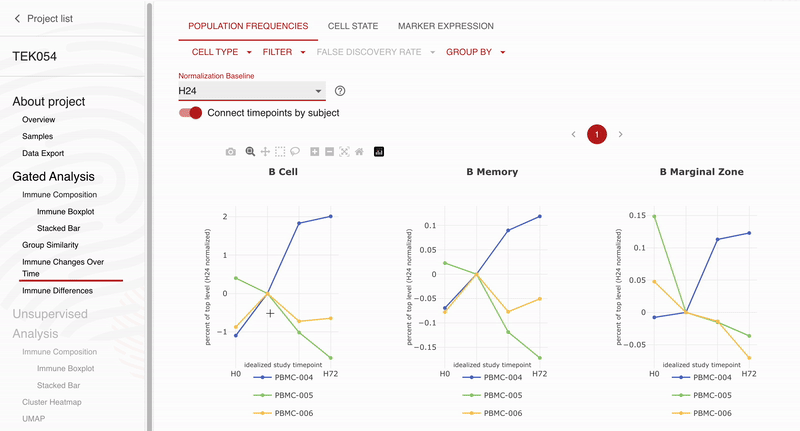
Teiko and Obsidian Therapeutics are excited to announce our partnership for high-dimensional immunophenotyping of patients enrolled in the ongoing OBX-115 phase 1 / 2 clinical trial. Teiko’s CLIA-validated 25-marker spectral flow cytometry panel will allow Obsidian to profile 298 immune cell subsets and states in each patient’s peripheral blood sample. The panel covers T cell, NK cell, B cell, and myeloid subsets along with 5 functional markers to evaluate maturation, exhaustion, and activation across these subsets. Obsidian will use Teiko’s service to analyze and describe peripheral blood immune compartments in patients participating in their phase 1 / 2 clinical trial.
“We selected Teiko for spectral flow immunophenotyping because of the strong data they have already generated for us in preclinical activities, and we are excited to continue to expand our partnership to explore the immune impacts of OBX-115 across the entire immune system,” Giri Ramsingh, Vice President, Clinical Sciences, Obsidian Therapeutics. “We especially appreciate the rigorous validation that Teiko’s panels have gone through and the easy-to-use data analysis tools that have allowed us to visualize our findings.”