
Video Webinar: Immune responses to cancer immunotherapy outside the tumor
Where do T cells get activated after cancer immunotherapy? Matthew Spitzer, PhD, reviews current literature and his lab’s recent Cell publication in this educational webinar.
Many blood cancer studies today use immune profiling detection platforms that simply count the number of cancer cells, without providing any detailed information about their characteristics or interactions with healthy immune cells. This is where mass cytometry comes in – with the ability to evaluate over 40 protein markers per cell across hundreds of thousands of cells per blood sample1, it provides high-dimensional data that researchers can use to make crucial therapeutic discoveries.
Here, we highlight six recent key discoveries made using mass cytometry across a wide range of blood cancers including acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), large B cell lymphoma, and multiple myeloma. With this powerful tool, researchers have uncovered new mechanisms of cancer relapse after chemotherapy, identified predictive biomarkers of response, and disentangled the role of non-cancerous immune cell populations in blood-based cancers.
Page Anchor: #chretien-2021 – Not Visible On FrontPublished in PNAS in 2021, Chretien et al. used two ~30 marker mass cytometry panels to characterize ‘dysfunctional’ natural killer (NK) cells overrepresented in newly diagnosed AML patients relative to healthy controls. This ‘dysfunctional’ NK population lacks expression of CD56, a marker highly associated with NK function, but expresses CD16, a crucial marker for antibody mediated killing by NK cells (CD56-CD16+).2 Importantly the cytotoxic killing potential of these cells, measured by degranulation in response to target cells, is reduced.
The authors found that having a higher percentage of these CD56- CD16+ NK cells at diagnosis was associated with poor clinical outcomes, lower overall survival, and a reduced likelihood of living free from disease complications (event-free survival). High dimensional analysis of single-cell protein expression by mass cytometry showed that these cells have lower expression of five activating receptors important for NK cytotoxic function: NKp30, NKp46, NKG2D, DNAM-1, and CD96.
Using an algorithm to analyze developmental pathways, the authors were able to identify a branching-off point where normal, mature NK cells diverge to become these dysfunctional NK cells. Future therapeutics targeting NK cells at this developmental stage may restore developmentally mature, functional NK cell populations for patients with AML, and thus likely improve clinical outcomes.
Page Anchor: #rorby-2021 – Not Visible On FrontA study by Rörby et al. published in late 2021 used mass cytometry to approach the question: how does PKC412 synergize with a current standard chemotherapy regimen to produce good responses in AML patients?3 PKC412 is a newer anti-cancer drug, also called midostaurin, that improves overall survival for AML patients treated with a standard chemotherapy regimen consisting of daunorubicin and cytarabine. In 2017, it received approval by the US Food and Drug Administration (FDA) for use in patients with newly diagnosed FLT3-mutated AML, which is a common mutation affecting a third of AML patients that carries a poor prognosis. The researchers wanted to parse out the mechanism behind PKC412’s synergy with standard chemotherapy in order to potentially achieve higher clinical response rates by boosting complementary pathways and eliminating antagonist ones.
To tease out the impact of PKC412 on each component of the conventional treatment regimen, Rörby et al. used a 37-marker mass cytometry panel to evaluate the effects of various combinations of PKC412, daunorubicin, and cytarabine on intracellular signaling.
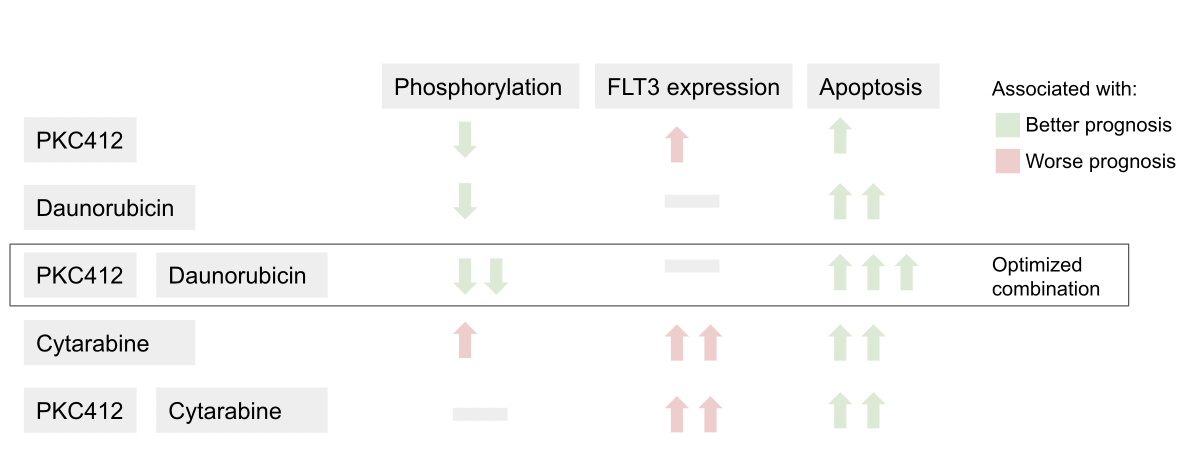
The authors found that daunorubicin works in concert with PKC412 to reduce phosphorylation (the addition of a phosphate group), a process that is necessary for molecular signaling cascades.3 In contrast, cytarabine increases FLT3 cell membrane receptor expression and intracellular signaling that reverses the activity of PKC412. These findings suggest that chemotherapeutic regimens without cytarabine, such as daunorubicin plus PKC412, may be more effective in treating the many AML patients who carry the FLT3-ITD mutation.
Page Anchor: #diaz-flores-2021 – Not Visible On FrontWith standard of care, around 90% of pediatric acute lymphoblastic leukemia (ALL) can be cured, but there is still around 10% of pediatric patients with relapsed or refractory (R/R) disease that remain resistant to treatment.4 A new drug that shows great potential to treat those with R/R ALL is Inotuzumab ozogamicin (InO), a CD22-directed antibody-drug conjugate. Although research from the Children’s Oncology Group and others suggested that resistance to InO might occur via reduced CD22 protein expression or density on the cell membrane, biomarkers for response to InO treatment had never been directly investigated.5
Diaz-Flores et al. used mass cytometry to evaluate whether the expression of any combination of 35 rationally selected protein antigens was associated with treatment response to InO.4 The proteins selected included cell surface markers, intracellular signaling molecules, and anti-apoptotic (cell survival) proteins that prevent programmed cell death, as well as other markers known to prevent cancer (so-called tumor suppressors) or resist drug exposure. After comparing antigen expression at diagnosis to changes in antigen expression after one and two cycles of treatment using a high-dimensional tumor cell-community clustering algorithm, the researchers were able to identify important combinatorial biomarkers predicting response to treatment.
The study authors identified the antigens that played the greatest role in distinguishing between complete and partial responder patient groups. Out of the handful of antigens identified, the most important discovery from the study was that low levels of CD22 had to be coupled with high levels of Bcl-2, an anti-apoptotic protein, to predict poor response to treatment. Low levels of CD22 alone could not predict poor response. These results suggested that the effects of InO, a drug that targets CD22, could possibly be improved by combining it with a drug to inhibit Bcl-2.
Page Anchor: #verkleij-2021 – Not Visible On FrontA study by Verkleij et al. used both flow and mass cytometry to evaluate the cellular response to the drug Daratumumab (DARA) in both newly diagnosed and relapsed/refractory multiple myeloma (R/RMM) patients.6 DARA, as part of a chemotherapy regimen, has been a promising treatment for R/RMM, with another study showing an improvement in complete response by 10% with the addition of DARA to a standard chemotherapy regimen.7 In this study, Verkleij et al. analyzed over 30 patient bone marrow and peripheral blood samples at baseline and at relapse using a mass cytometry panel with 39 surface and intracellular markers.
The authors found greater numbers of NK cells with an exhausted phenotype (higher frequency of TIM-3 expression and lower frequency of CD16 expression) in non-responding patients compared to responding patients at baseline, which was also seen in patients who relapsed. NK cells with an exhausted phenotype no longer carry out their normal characteristic functions such as the release of inflammatory signals or the ability to kill cancer cells. Patients with poor clinical outcomes also had greater numbers of both NK and T cells with an exhausted phenotype.
To determine whether resistance to DARA was due to NK cell exhaustion rather than a change in the multiple myeloma cancer cells themselves, the authors took cancer cells from patients who developed resistance to DARA and combined them with NK cells from healthy donors plus DARA treatment. They saw that DARA resistance was overcome by the addition of healthy NKs, suggesting that patients resistant to DARA may benefit from targeted reinvigoration of NK cells so they can destroy cancer cells again. This insight into the key role of NK cell activity was made possible by large-scale immune profiling through mass cytometry and is broadly applicable to situations where the target immune cell is initially unknown.
Page Anchor: #melnekoff-2021 – Not Visible On FrontAnother promising treatment for R/RMM is B cell maturation antigen (BCMA) CAR-T cell therapy, which involves genetically engineering a patient’s T cells so that they respond more forcefully to combat cancer cells. While many patients respond well to therapy, the response can be quite variable both in terms of initial effectiveness and in the duration of benefit. To explore variables that predict how patients will respond to BCMA CAR-T cell therapy, Melnekoff et al. conducted a study assessing single-cell transcriptomic and proteomic data from bone marrow (which is the tumor microenvironment in multiple myeloma) and peripheral blood samples using mass cytometry and single-cell RNA sequencing (scRNA-seq).8, two complementary technologies.
Using a mass cytometry panel of 39 cell markers, study authors analyzed 3.5 million peripheral blood mononuclear cells (PBMCs) from 11 patients treated with BCMA CAR-T cell therapy. Analysis of samples taken from peripheral blood and the bone marrow tumor microenvironment at baseline revealed that there were distinct cell population markers distinguishing good responders from poor responders, with responders having greater percentages of CD8+ T cells and lower percentages of CD14+ monocytes in both peripheral blood and bone marrow and lower NK cell population percentages in peripheral blood.
These same population changes were also observed over time in response to expansion of CAR-T cells in patients. Comparing week 0 to week 4 on CAR-T therapy, non-engineered CD8+ T cell numbers increased while CD14+ monocyte numbers decreased. The non-engineered CD8+ T cells were found to be exhibiting a so-called “effector-memory” phenotype that enables an immediate robust immune response at the site of contact with the disease-carrying cells. These shifts reverse when a relapse on CAR-T therapy occurs. CD14+ monocyte numbers level off or increase and CD8+ T cell numbers decline. Melnekoff et al. concluded that a certain class of immunomodulatory drugs called immunomodulatory imide drugs might increase the effectiveness of CAR-T therapy by increasing natural CD8+ T cell numbers.
Page Anchor: #good-2022 – Not Visible On FrontTreating diffuse large B cell lymphoma (DLBCL) patients with axi-cel, a chimeric antigen receptor (CAR) T cell therapy targeting CD19 (an important B cell biomarker) has proven to be difficult, with lymphoma continuing to progress in around 60% of patients and neurological toxicity affecting two-thirds of patients.9 Biomarkers connected to poor disease control and neurotoxicity have not been well characterized. For this reason, Good et al. conducted a study to investigate circulating CAR T cells in 32 patients treated with axi-cel using mass cytometry to profile 37 proteins per cell.
The researchers found two metaclusters associated with complete response, both of which were positive for CD57 and expressed inhibitory proteins, such as PD-1, at lower levels.9 They also found a metacluster of CD4+Helios+ CAR T cells 7 days post-infusion that correlated with disease progression and diminished neurotoxicity. These cells expressed high levels of CD25, CTLA4 and TIGIT, a phenotype characteristic of immunosuppressive regulatory T cells (Tregs). The authors validated their findings in a prospective cohort analysis of 31 DLBCL patients treated with axi-cel.
This study demonstrates the usefulness of mass cytometry to find biomarkers correlated with treatment response, disease progression, or toxicity in DLBCL patients. The discovery of a unique population of CAR Treg cells associated with disease progression and reduced neurotoxicity may allow future researchers to specifically manipulate these cells before or after infusion to promote anti-tumor effect or lower neurotoxicity.
In addition to the examples highlighted earlier, recent research presented at the December 2022 American Society of Hematology Conference investigated many other blood cancers, including follicular lymphoma and chronic myeloid leukemia. Other ongoing studies focus on AML patients with adverse cytogenetics and drug resistance in patients with other blood cancers like DLBCL and multiple myeloma.
Collectively, these studies showcase the capabilities of mass cytometry to identify peripheral immune biomarkers that can predict relapse and treatment response across a broad range of blood cancers. A recent pre-print by Kleftogiannis et al. does just this using a combination of mass cytometry and machine learning algorithms to identify predictors of survival in leukemia. The technology’s unique ability to profile both the patient’s tumor and immune cells at the single-cell protein level positions it as an excellent tool to unravel the mechanisms behind relapse and response. This deeper understanding of the disease can then inform the development of more effective treatment protocols and therapies.