Customizable Clinical Cytometry Panels
Add additional markers to a verified cytometry backbone to profile specific cell types, targets, and pathways, relevant to your research and drug programs.
TokuProfile backbone panels are constructed based on extensive prior knowledge, input from our academic and pharma partners, and years of experience over the course of multiple mass cytometry projects.
Each panel includes 1-10 open channels for markers to detect target-specific biomarkers and understand the mechanism of action, dose selection, and drug pharmacodynamics.
Panel Verification Report
Custom high-dimensional cytometry panels tailored to your drug programs.With the development of a custom cytometry panel, customers will receive a panel verification report that includes:
- The clone, conjugated metal, and optimal staining concentration for each antibody included in the panel.
- A six-point titration for each antibody added to the panel backbone will be performed, and the following performance metrics will be reported:
- Staining Index or Arcsinh Ratio
- Frequencies of positive and negative cell populations displayed as a dot plot or histogram at each antibody concentration
- Spillover (as a percent frequency) into nearby (+1, -1) and relevant (+16) metal channels on the panel
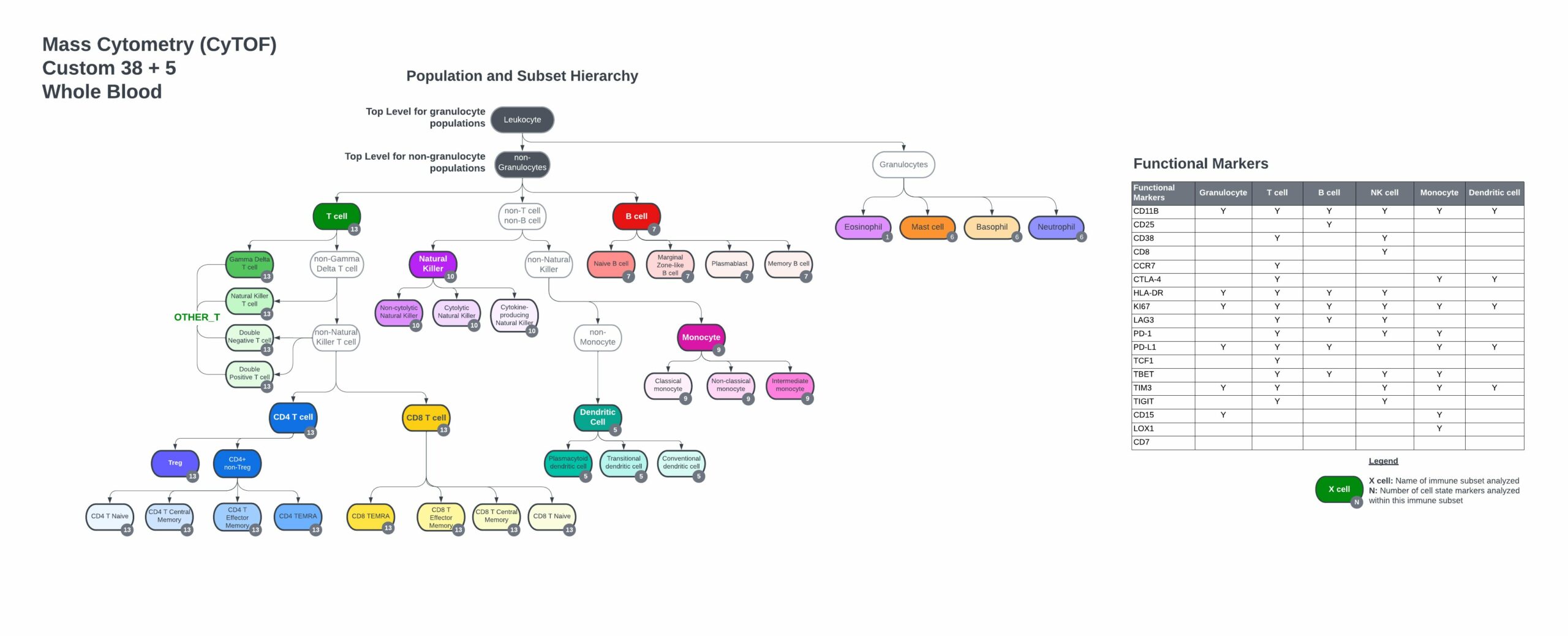
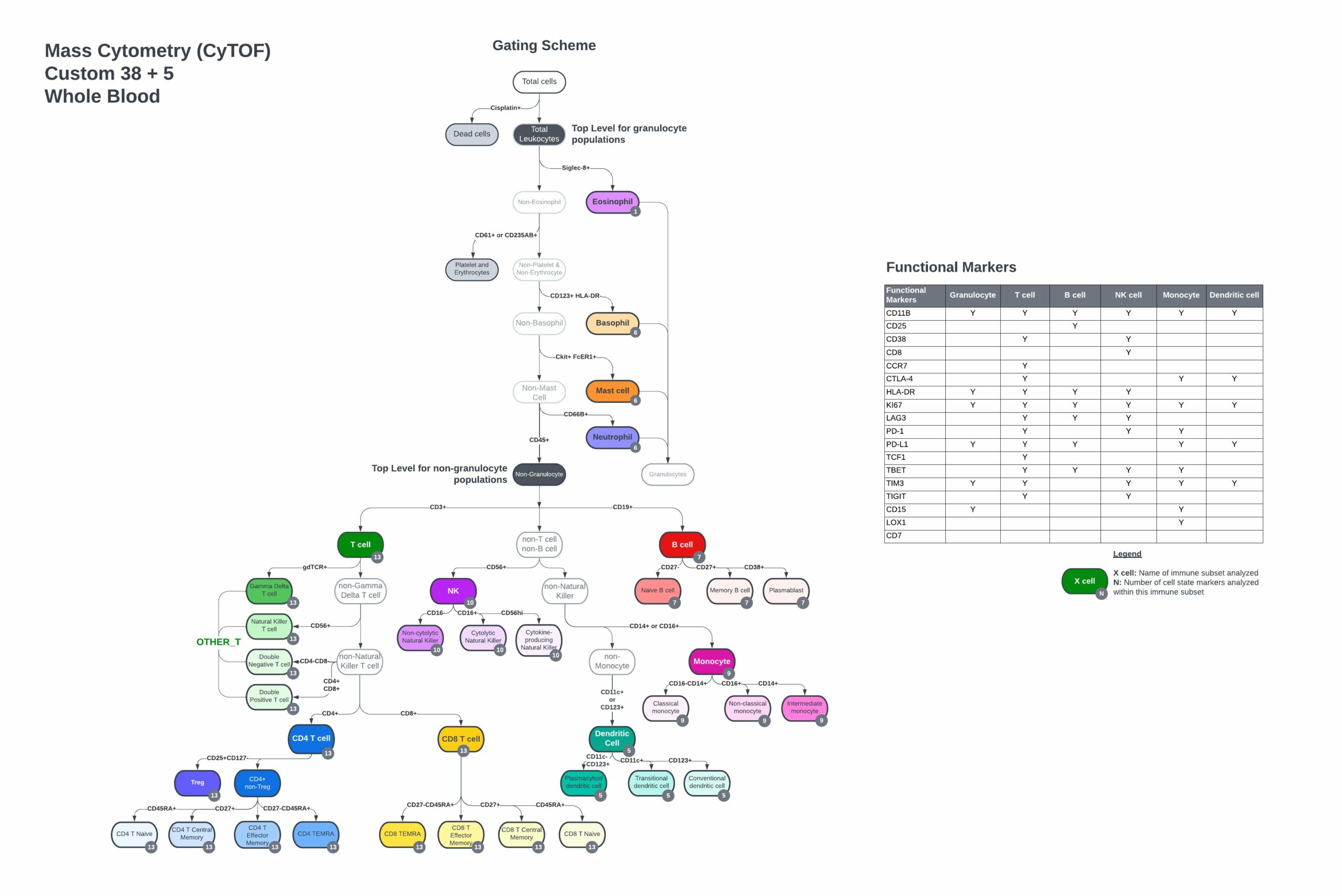
- Complete gating strategy, including immune cell populations and functional marker subsets from control PBMC or whole blood samples
Customizable Panel Designed by Experts
The TokuProfile Human Pan-Immune Panel Backbone includes:
- Markers to identify every major immune cell population and subsets.
- Expression of key co-stimulatory and co-inhibitory molecules that regulate cell functions.
- Markers of recent activation and effector function.
- Up to 10 open channels for customization.
- Customize the backbone to your whole blood sample with 5 pre-selected antibodies, Siglec-8, FcER1, CD15, LOX-1, and cKit, to profile eosinophils, neutrophils, basophils, mast cells, and polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs).
Custom PBMC panel immune hierarchy and gating strategy
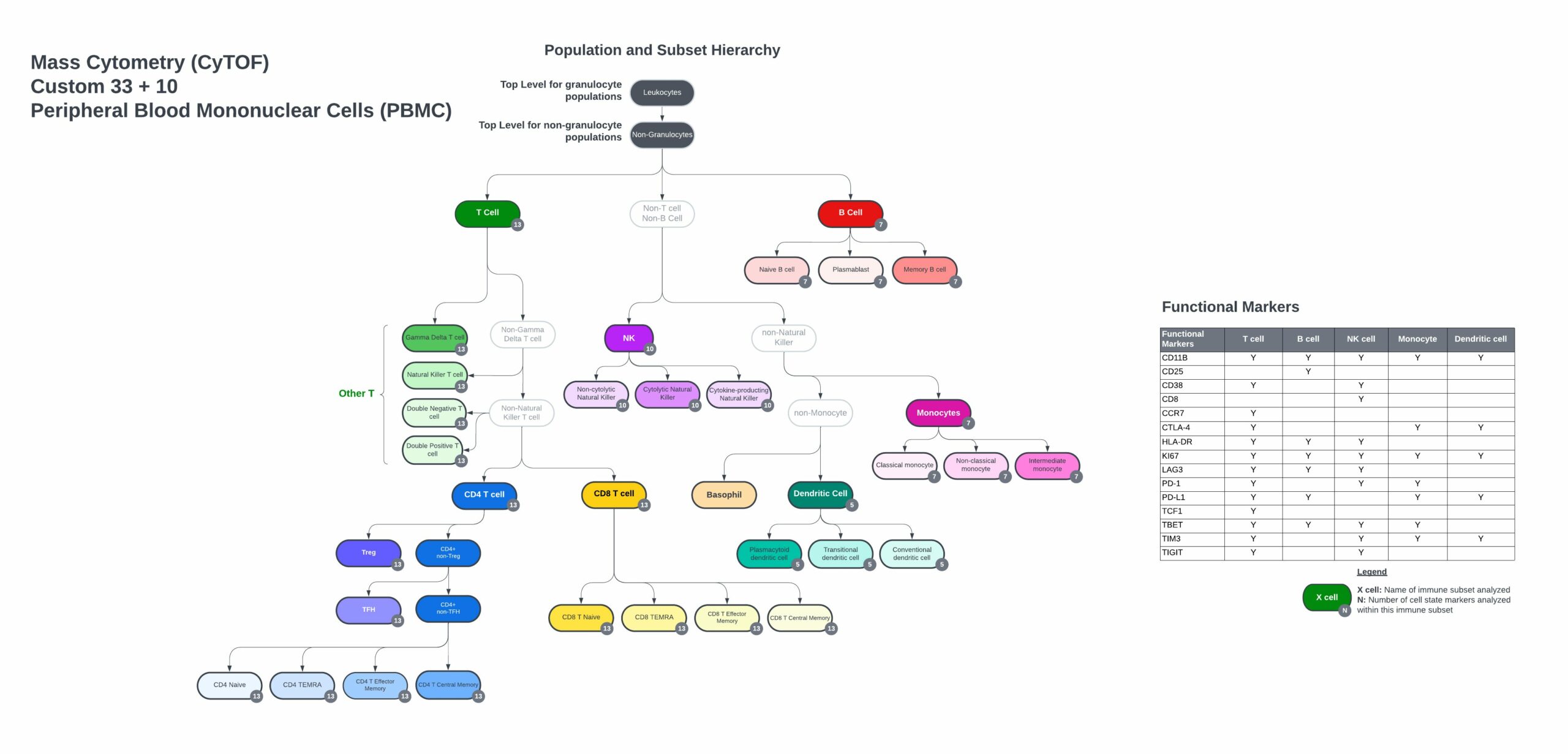
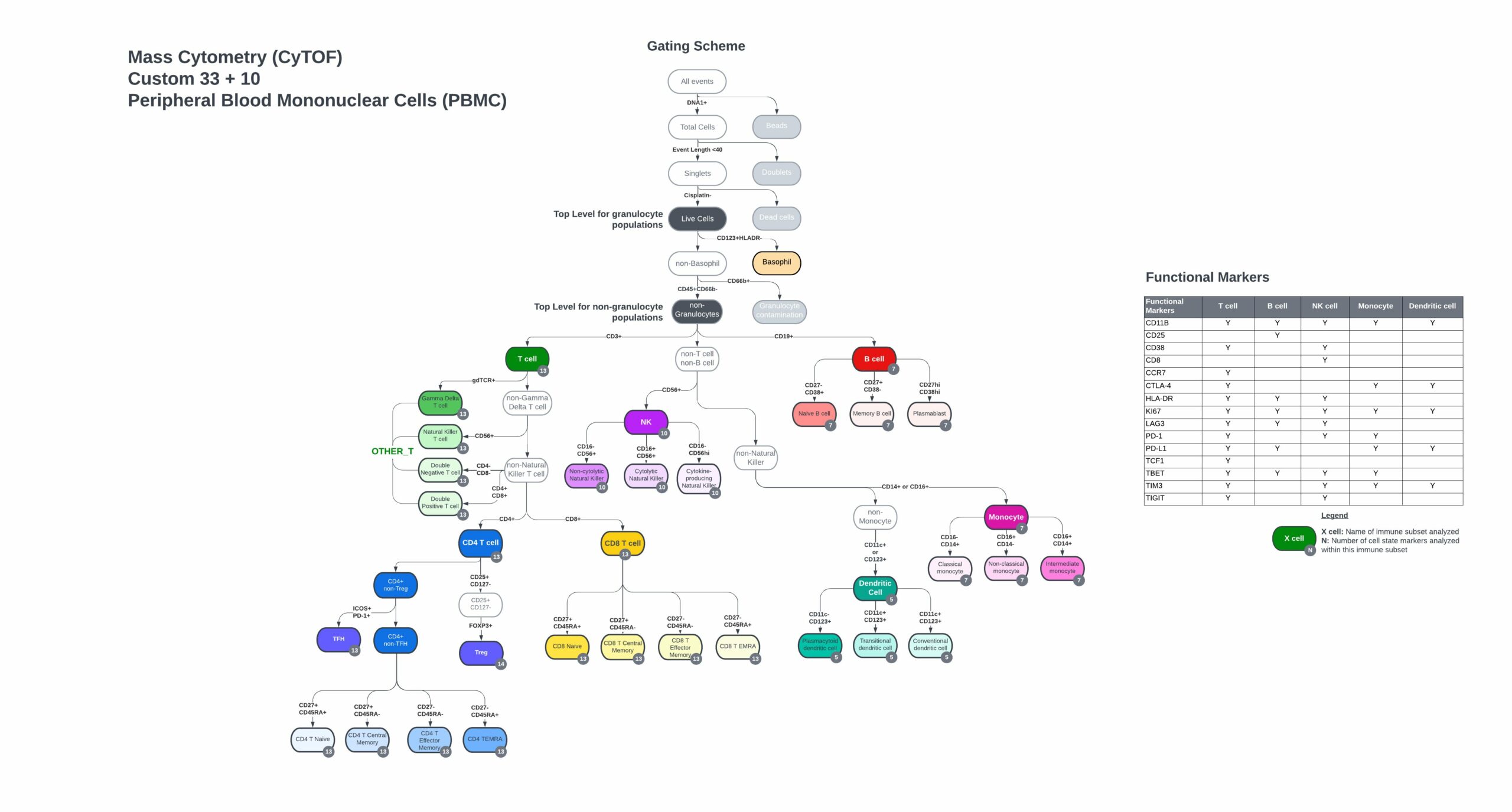
33 markers with 10 open channels
Suggested custom whole blood gating scheme