CLIA-Validated Whole Blood Mass Cytometry Immune Profiling Assay
Clinical trials-grade immunophenotyping with 40+ markers for whole blood immune detection
Identify immune biomarkers for dose selection, mechanism of action, and pharmacodynamic studies in your clinical trials.
Analyze up to 735 immune cell subsets and states with our 41-marker panel. Accurate subset measurements with <10% coefficient of variation for all major immune cell types.
Total Average Coefficient of Variation:
- Intra-Run Precision: 2.18%
- Inter-Run Precision: 6.84%
- Inter-Kit Precision: 5.75%
Validated with our TokuKit – designed for simplified sample collection at clinical sites.
Up to 720+ Immune Subsets and States Analyzed
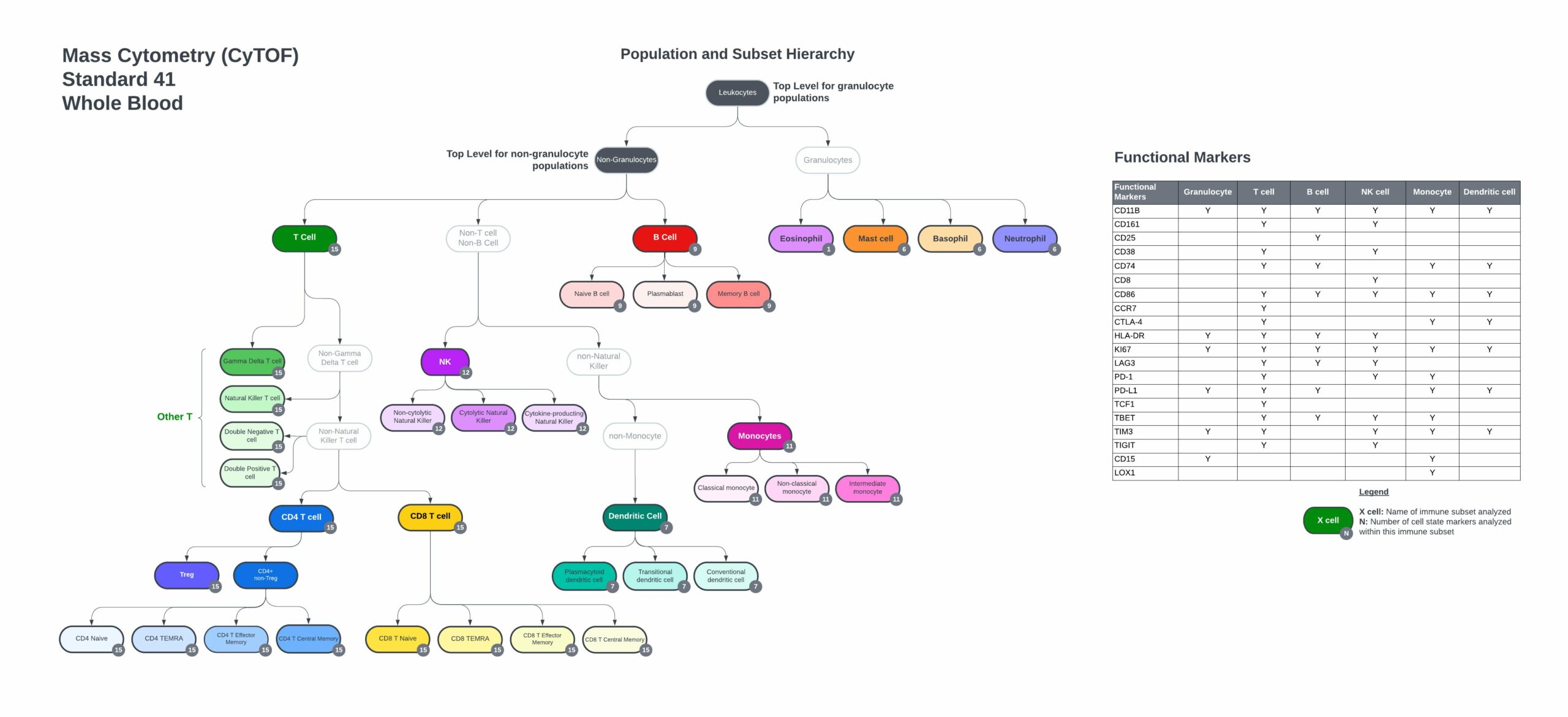
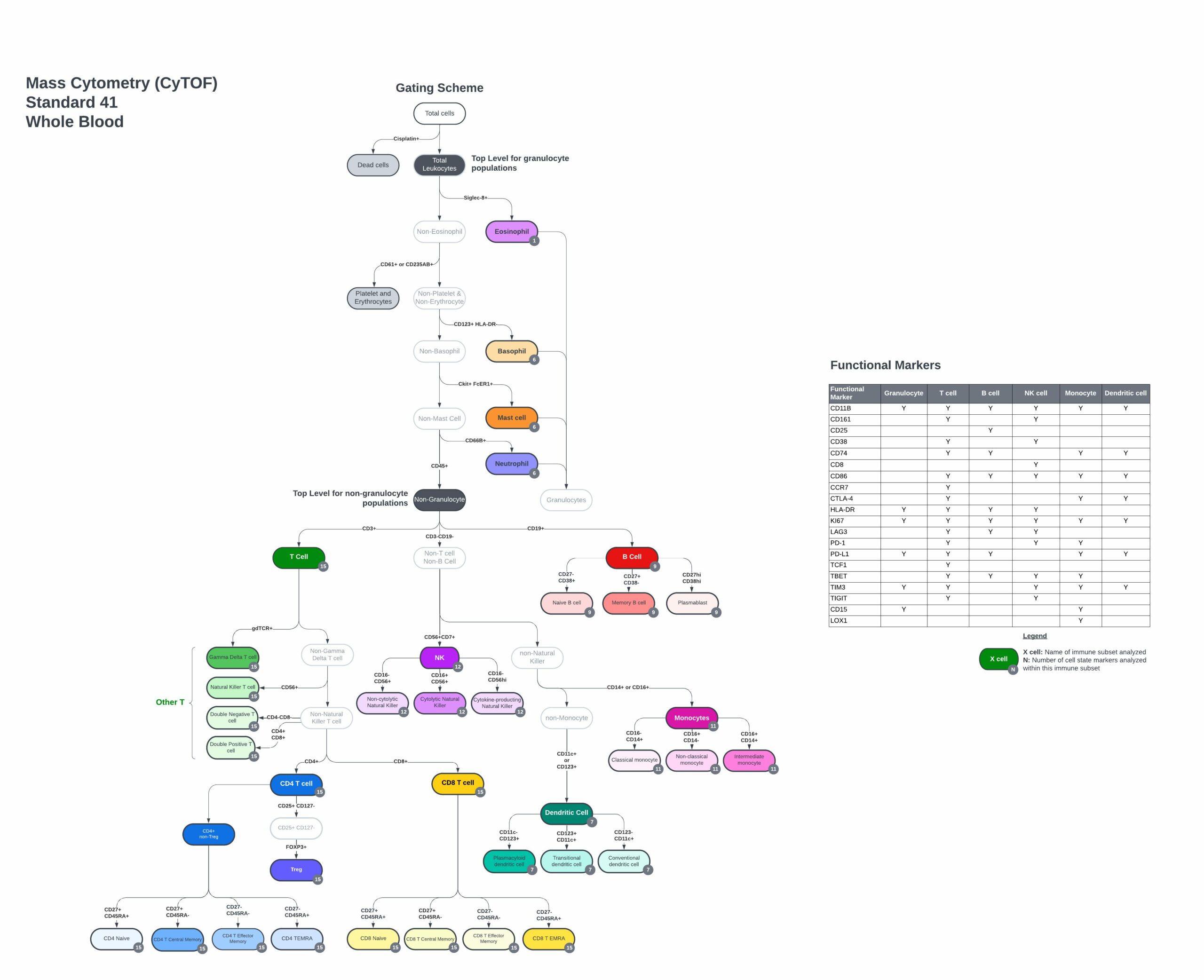
Expertly Designed Human Pan-Immune Whole Blood Panel
The TokuProfile Standard Human Pan-Immune Whole Blood panel includes:
- Markers to identify every major immune cell population and subsets.
- Expression of key co-stimulatory and co-inhibitory molecules that regulate cell function.
- Markers of recent activation and effector function.
- Compatible with the TokuKit – Teiko’s whole blood collection kit.
Markers to define cell populations and quantify functional states
Coefficient of Variation (CV) Among Immune Cell Subsets
Precision and Median Cell Count Data
* = No CV calculated (subset was below 100-cell minimum in >1 replicate)

CLIA Validation Data
Mass Cytometry Assay for Pan-Immune Profiling of Whole Blood Collected with TokuKit-SHFill out the form below to access our CLIA Validation plan, report, data and SOPs.