- Products & Services TokuProfile Spectral Flow CytometryWhole Blood KitTokuProfile Mass CytometryData
- Resources
- Pricing
- Company
- Login
Articles
13 markers should be good enough for my flow panel, right?
Ramji Srinivasan 6/11/24
“I have a 13 marker panel that analyzes T-cells. And my drug just hit the T-cells, why bother looking at any other immune cell types, like B cells, or NK cells? Even if those other cells are affected, I don’t know how to interpret those results.”
The reason is: those extra subsets might predict the difference between success and failure for a drug.
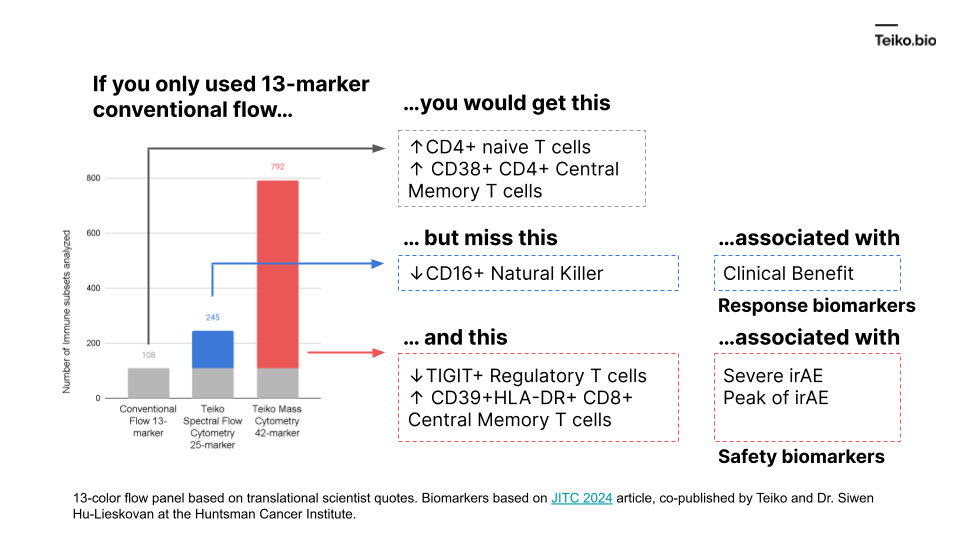
More to the point, if we took a conventional flow panel, you get up to ~108 subsets with a 13-marker panel, almost exclusively T cells. Using mass cytometry, with a 42-marker panel, you get to 792. With spectral flow, it’s around 245. This diagram shows what is missed by the lower-parameter methods. And these are cell subsets we ourselves have found in our own work and collaborations, associated with either clinical benefit, severe immune-related adverse events or peak toxicity.
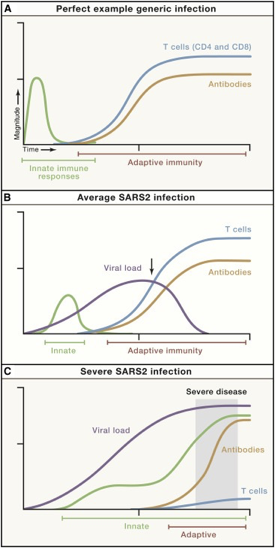
We know there is a “characteristic” time-series curve associated with clearing infections, like COVID. It would be difficult for a drug developer to analyze a “productive” immune response if whole curves were missing. For example, imagine tracking one curve, like the antibodies. If you tracked just antibodies, you could be misled into thinking someone had an average COVID infection or generic infection.
There is good reason to believe this sort of curve is true in cancer immunology. To that point, this is a nice time-series diagram from Nature Medicine of different cell types in the tumor microenvironment. The authors summarized it well: “understanding what cell types can be modulated and when may enable the next biggest improvements in immunotherapy.”
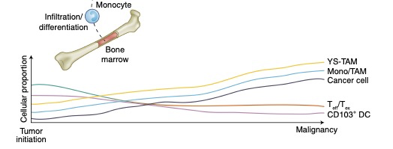
These distinct subtypes are not of academic interest: these drive real-world responses.